What is EPCS?
The Practice Fusion electronic health record (EHR) provides advanced electronic prescribing capabilities, including electronic prescribing of controlled substances (EPCS*).
Start a 14-day free trial to get started.
EPCS Definition
Electronic prescribing of controlled substances, or EPCS, is the process of electronically transmitting prescriptions for controlled substances to the pharmacy. Prescribers are encouraged to write and transmit prescriptions for controlled substances electronically to increase safety and security, and to reduce risk of fraud and diversion.1 Pharmacists are then permitted to receive, dispense, and archive the electronic prescriptions sent to them by the prescriber.2
Controlled Substance Prescription
Controlled substances are drugs that have the potential for abuse, misuse, and dependence.3 These drugs are regulated by the federal Controlled Substance Act (CSA) to protect public health and safety. The CSA divides controlled substances into five categories, known as schedules. They are assigned specific schedules based on whether they have a currently accepted medical use in the United States, their relative potential for abuse, and the risk of causing dependence if they are abused.4-6
| Schedule | Description |
|---|---|
| Schedule I | Substances in this schedule have no currently accepted medical use in the United States and a high potential for abuse. They do not have accepted safety for use under medical supervision.5 Examples: Heroin, lysergic adic diethylamide (LSD), 3,4-methylenedioxymetham-phetamine (“Ectasy”)5 Marijuana (cannabis) is classified as a schedule I controlled substance by the federal government, but some states have passed laws legalizing medical and recreational use7 |
| Schedule II |
Substances in this schedule have a high potential for abuse with severe psychological or physical dependence.5 Examples Schedule II narcotics: hydromorphone (Diaudid®), methadone, oxycodone (OxyContin®, Percocet®), fentanyl (Sublimaze®, Duragesic®), morphine, opium, codeine, hydrocodone5 Examples Schedule IIN stimulants: amphetamine (Dexedrine®, Adderall®), methamphetamine (Desoxyn®), methylphenidate (Ritalin®)5 |
| Schedule III | These substances have a lesser potential for abuse than schedules I or II controlled substances. Their abuse may lead to moderate or low physical dependence or high psychological dependence.5 Examples Schedule III narcotics: products containing not more than 90 mg of codeine per dosage unit (Tylenol with Codeine®), buprenorphine (Suboxone®)5 Examples of Schedule III non-narcotics: benzphetamine (Didrex®), phendimetrazine, ketamine, anabolic steroids5 |
| Schedule IV | These substances have a low potential for abuse relative to substances in schedule III.5 Examples: alprazolam (Xanax®), carisoprodol (Soma®), clonazepam (Klonopin®), clorazepate (Tranxene®), diazepam (Valium®), lorazepam (Ativan®), midazolam (Versed®), temazepam (Restoril®), triazolam (Halcion®)5 |
| Schedule V | These substances have a low potential for abuse relative to substances in schedule IV. They are made up primarily of preparations containing limited amounts of narcotics.5 Examples: cough preparations containing not more than 200 mg of codeine per 100 ml or per 100 grams (Robitussin AC®, Phenergan with Codeine®), ezogabine5 |
EPCS History
In 2010, the Drug Enforcement Agency (DEA) passed a new rule allowing providers to prescribe controlled substances electronically through a certified system. EPCS was seen as a way to reduce some of the issues created by paper prescriptions, such as forged, stolen, or illegible prescriptions, by requiring authentication of prescribers, improving security standards, and auditing activity on EPCS platforms.1
Today, numerous factors drive mandated implementation of e-prescribing of controlled substances in individual states. For many, EPCS has been mandated as an active step in the fight against prescription opioid misuse and abuse. It provides a structured format for monitoring and oversight of patients’ prescription histories and controlled substance prescriptions, which significantly reduces opportunities for misuse and abuse of controlled substances.4
Provider Responsibilities
Under DEA Title 21, the provider is responsible for the safety of EPCS. Before utilizing EPCS to prescribe controlled substances, the provider is required to register with the DEA so that individuals who are not registered will be unable to gain access to the applications used for electronically issuing prescriptions. The provider is also required to have his or her identity verified in order to obtain two-factor authentication tokens, for use when issuing electronic prescriptions.8 The provider must retain sole possession of the authentication token(s), password, and other sign-on information and must not share this information with any other person or prescribing entity.
The provider has the same responsibilities when issuing a prescription for a controlled substance by paper, voice, or electronic means. As when delivering a paper or oral prescription, the provider is required to issue electronic prescriptions for controlled substances only for legitimate medical purposes and only as part of a professional medical practice. The electronic prescription is required to include the same information as paper or oral prescriptions, including the date the prescription was issued, the patient’s full name, the medication name, dosage strength and form, quantity prescribed, directions for use, earliest date when the pharmacy may refill the prescription, and the name, address, and DEA registration number of the prescribing physician.9
EHR Technology Requirements
Under DEA Title 21, providers are required to use an electronic prescribing technology that has been certified by a third-party auditor or certification organization. The provider must obtain identity verification through the electronic prescribing application and use two-factor authentication to issue, sign, and refill prescriptions for controlled substances.
EPCS Mandates
Federal Mandate: Medicare Part D and Medicare Advantage Prescription Drug Plan Prescribers
In 2018, the federal government passed the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT Act). This Act was passed as a response to the nation’s opioid crisis. It requires that prescriptions for controlled substances covered under Medicare Part D or Medicare Advantage Prescription Drug Plans be submitted electronically beginning January 1, 2021. Medicare Advantage and Medicare Part D prescribers in all states are subject to this mandate and must comply.2,10,11
However, in the face of the COVID-19 national health emergency, the Centers for Medicare and Medicaid Services (CMS) announced that it would delay enforcement of penalties until January 1, 2022.12-14
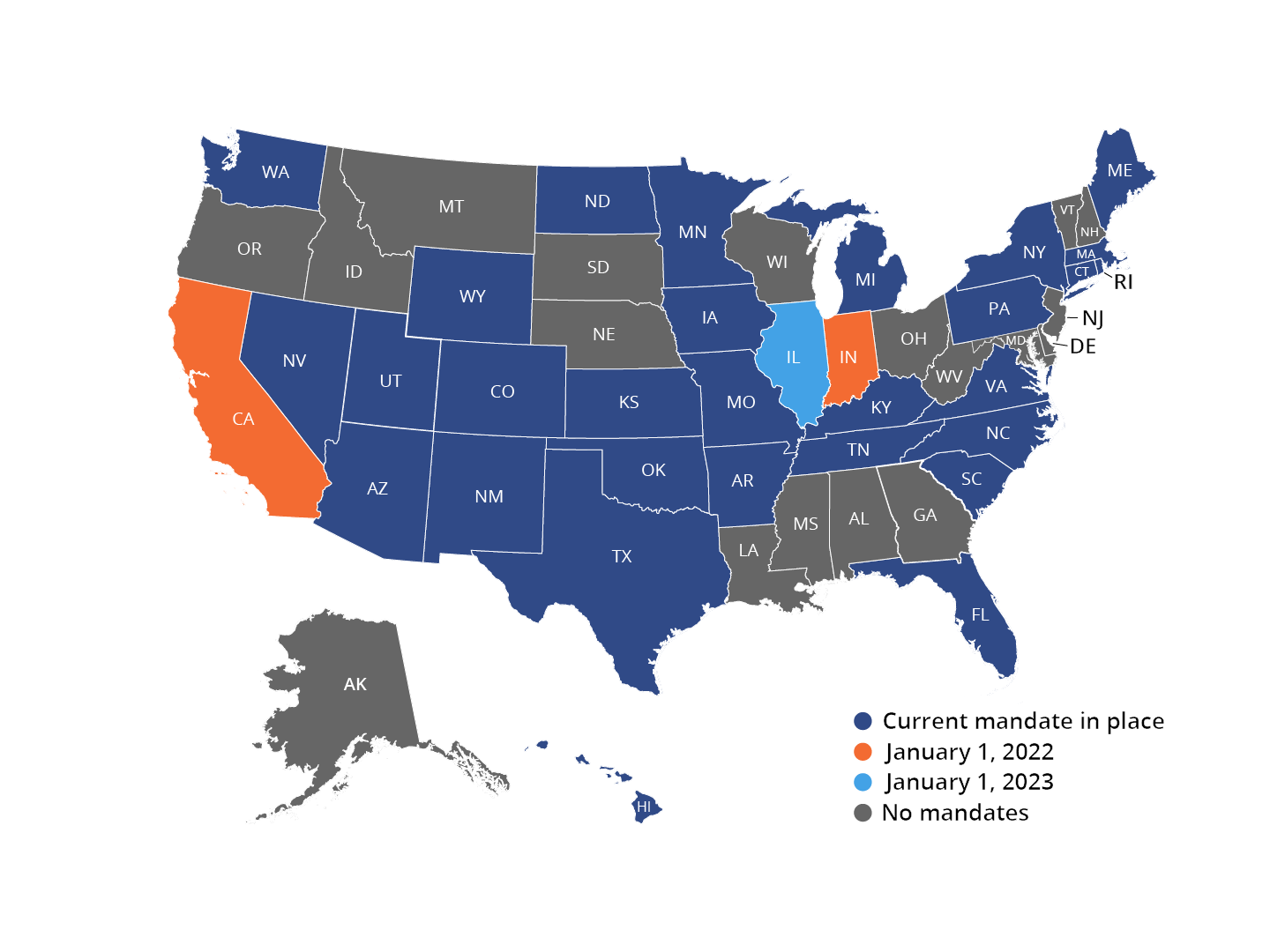
State EPCS Mandates
Although the CMS will not enforce penalties for non-compliance with its EPCS mandate until January 1, 2022, many states have already mandated EPCS.14 Currently, twenty-eight states have EPCS mandates. Numerous additional states have set future implementation dates for EPCS mandates.15
Individual states’ mandates may differ in the specific schedules of controlled substances that must be electronically prescribed. Some states require all prescriptions to be electronically prescribed, including both controlled and non-controlled substances.15 To ensure compliance, it is essential for prescribers to confirm the details of their specific states’ EPCS requirements.
For more information on the details of each state’s EPCS mandate status, please refer to the individual state links below.
Prescription Drug Monitoring Programs (PDMPs)
Currently, Practice Fusion provides the means to use EPCS in all fifty states and the District of Columbia. In addition to the requirement for EPCS, many states have also implemented mandates requiring physicians and pharmacists to check PDMPs prior to prescribing or dispensing controlled substances. A PDMP is a state-run electronic database that tracks all prescriptions for controlled substances in the area. PDMPs exist to help monitor how opioids and other controlled substances are prescribed, with the goal of helping to reduce the number of people who are misusing or abusing these medications. Currently forty-six states have mandates for PDMP utilization.18
Many states that mandate PDMP use have also enabled PDMP integration with individual practices’ EHR and EPCS systems.18 Requirements for integration vary from state to state. Practice Fusion is currently able to integrate the following six states’ PDMP systems:
EPCS Updates
Practice Fusion’s EPCS feature is fully certified and ready for use in all fifty states and the District of Columbia. With EPCS activated, prescribers will be able to e-prescribe most medications, including scheduled controlled substances, directly from Practice Fusion’s EHR.
Get started with our certified EPCS system
An EPCS system can be costly to maintain for each provider every year to stay in compliance. However, electronic prescribing, including EPCS, is included in your Practice Fusion subscription at no additional charge.
14-day free trial
Experience first-hand the value of a Practice Fusion subscription. There’s no risk, no commitment, and no cost or credit card required.
References:
- Opioid Epidemic & Health IT. The Office of the National Coordinator for Health Information Technology. Updated December 18, 2019. Accessed September 16, 2021, https://www.healthit.gov/playbook/opioid-epidemic-and-health-it/.
- Electronic Prescribing of Controlled Substances (EPCS) among Office-Based Physicians, 2017. HealthIT.gov. Updated September 2019. Accessed September 16, 2021, https://www.healthit.gov/data/data-briefs/electronic-prescribing-controlled-substances-epcs-among-office-based-physicians.
- Controlled substance. National Cancer Institute (NCI). Accessed September 20, 2021, https://www.cancer.gov/publications/dictionaries/cancer-terms/def/controlled-substance.
- TheraNest Team. Most Common Psychiatric Controlled Substances. TheraNest. Updated November 23, 2020. Accessed June 15, 2021, https://theranest.com/blog/most-common-psychiatric-controlled-substances/.
- Controlled Substance Schedules. U.S. Department of Justice Drug Enforcement Administration - Diversion Control Division. Accessed September 20, 2021, https://www.deadiversion.usdoj.gov/schedules/.
- 79 Electronic Prescriptions for Controlled Substances: Notice of Approved Certification Process (The Federal Register Online via the Government Printing Office) 73907.
- Schedule I controlled substance. National Cancer Institute. Accessed September 29, 2021, https://www.cancer.gov/publications/dictionaries/cancer-terms/def/schedule-i-controlled-substance.
- 85 Electronic Prescriptions for Controlled Substances (Diversion Control Division) 22018-22021 (April 21, 2020).
- Title 21 Code of Federal Regulations, Part 1311–Requirements for Electronic Orders and Prescriptions (U.S. Department of Justice: Drug Enforcement Administration).
- Centers for Medicare & Medicaid Services (CMS). Medicare Program: Electronic Prescribing of Controlled Substances; Request for Information (RFI). Federal Register: The Daily Journal of the United States Government. Updated August 4, 2020. Accessed September 16, 2021, https://www.federalregister.gov/documents/2020/08/04/2020-16897/medicare-program-electronic-prescribing-of-controlled-substances-request-for-information-rfi.
- The SUPPORT for Patients and Communities Act (P.L.115-271): Medicare Provisions. In: Service CR, editor. R45449. January 2, 2019 ed2019.
- CMS Delays Federal EPCS Compliance Enforcement To 2022. RXNT. Updated February 17, 2021. Accessed August 9, 2021, https://www.rxnt.com/cms-delays-epcs-compliance-enforcement-to-2022/.
- CMS formally delays EPCS enforcement. National Community Pharmacists Association. Updated December 4, 2020. Accessed August 22, 2021, https://ncpa.org/newsroom/qam/2020/12/04/qam-ad-cms-formally-delays-epcs-enforcement.
- CMS delays enforcement of e-prescribing requirement for controlled substances. California Medical Association. Updated December 15, 2020. Accessed September 16, 2021, https://www.cmadocs.org/newsroom/news/view/ArticleId/49150/CMS-delays-enforcement-of-e-prescribing-requirement-for-controlled-substances.
- State PDMP Profiles and Contacts. Prescription Drug Monitoring Program Training and Technical Assistance Center. Updated July 7, 2021. Accessed August 19, 2021, https://www.pdmpassist.org/State.
- SB19-079 Electronic Prescribing Controlled Substances. Colorado General Assembly. Updated April 8, 2019. Accessed September 17, 2021, https://leg.colorado.gov/bills/sb19-079.
- Illinois HB3596 Controlled Substances–Opioids. Trackbill. Updated August 20, 2021. Accessed September 17, 2021.
- What is a PDMP (Prescription Drug Monitoring Program)? Practice Fusion. Updated September 1, 2021. Accessed September 16, 2021, https://www.practicefusion.com/blog/what-is-a-pdmp/.
*Electronic Prescriptions for Controlled Substances (EPCS) is only available in the 50 US states and the District of Columbia
