EPCS Overview
Electronic prescribing of controlled substances, or EPCS, is the process of electronically transmitting prescriptions for controlled substances to the pharmacy. In 2010, the Drug Enforcement Agency (DEA) passed a new rule allowing providers to prescribe controlled substances electronically through a certified system.1
EPCS is an important tool in the fight against prescription opioid misuse and abuse as it provides a structured format for monitoring and oversight of patients’ prescription histories and use of controlled substances. EPCS makes it easier for both providers and pharmacists to identify patients who might be “doctor shopping” or show other signs of prescription drug abuse or misuse.1
By 2015, all fifty states had updated their regulations to make EPCS legal. However, although EPCS is legal in all fifty states, it is not yet widely adopted. The majority of prescribers are sending electronic prescriptions, but only an average of 23.6% of U.S. practitioners are currently able to prescribe controlled substances electronically.1
Federal Mandate: Medicare Part D and Medicare Advantage Prescription Drug Plan Prescribers
In 2018, the federal government passed the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT Act). This Act was passed as a response to the nation’s opioid crisis. It requires that prescriptions for controlled substances covered under Medicare Part D or Medicare Advantage Prescription Drug Plans be submitted electronically beginning January 1, 2021. Medicare Advantage and Medicare Part D prescribers in all states are subject to this mandate and must comply.2-4 However, in the face of the COVID-19 national health emergency, the Centers for Medicare and Medicaid Services (CMS) announced that it would delay enforcement of penalties until January 1, 2022.5-7
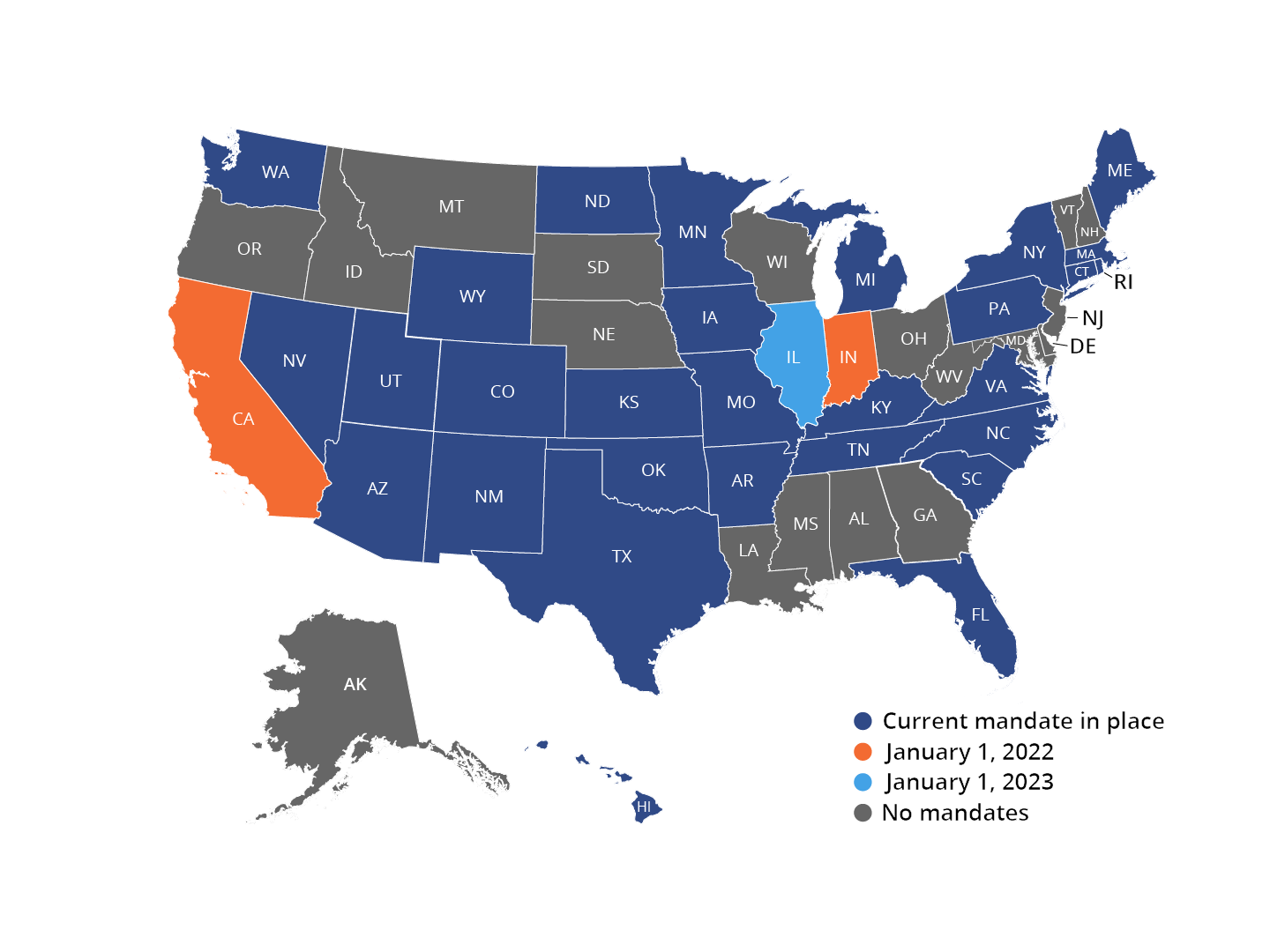
State EPCS Mandates
Although CMS will not enforce penalties for non-compliance with its EPCS mandate until January 1, 2022, many states have already mandated EPCS.7 As of October 2021, twenty-eight states have EPCS mandates. Numerous additional states have set future implementation dates for EPCS mandates.8
Individual states’ mandates may differ in the specific schedules of controlled substances that must be electronically prescribed. Some states require all prescriptions to be electronically prescribed, including both controlled and non-controlled substances.8 To ensure compliance, it is essential for prescribers to confirm the details of their specific states’ EPCS requirements. For more information on the details of each state’s EPCS mandate status, please refer to the individual state links below.
Prescription Drug Monitoring Programs (PDMPs)
Currently, Practice Fusion provides the means to use EPCS in all fifty states and the District of Columbia. In addition to the requirement for EPCS, many states have also implemented mandates requiring physicians and pharmacists to check PDMPs prior to prescribing or dispensing controlled substances. A PDMP is a state-run electronic database that tracks all prescriptions for controlled substances in the area. PDMPs exist to help monitor how opioids and other controlled substances are prescribed, with the goal of helping to reduce the number of people who are misusing or abusing these medications. Currently forty-six states have mandates for PDMP utilization.11
Many states that mandate PDMP use have also enabled PDMP integration with individual practices’ electronic health record (EHR) and EPCS systems.11 Requirements for integration vary from state to state. Practice Fusion is currently able to integrate with these six states’ PDMP systems:
EPCS Updates
Practice Fusion’s e-prescribing for controlled substances (EPCS) feature is certified and ready for use in all fifty states and the District of Columbia. With EPCS activated, prescribers will be able to e-prescribe most medications, including scheduled controlled substances, directly from the Practice Fusion EHR.
Get started with our certified EPCS system
An EPCS system can be costly to maintain for each provider every year to stay in compliance. However, electronic prescribing, including EPCS, is included in your Practice Fusion subscription at no additional charge.
14-day free trial
Experience first-hand the value of a Practice Fusion subscription. There’s no risk, no commitment, and no cost or credit card required.
References:
- Opioid Epidemic & Health IT. The Office of the National Coordinator for Health Information Technology. Updated December 18, 2019. Accessed September 16, 2021, https://www.healthit.gov/playbook/opioid-epidemic-and-health-it/.
- Centers for Medicare & Medicaid Services (CMS) H. Medicare Program: Electronic Prescribing of Controlled Substances; Request for Information (RFI). Federal Register: The Daily Journal of the United States Government. Updated August 4, 2020. Accessed September 16, 2021, https://www.federalregister.gov/documents/2020/08/04/2020-16897/medicare-program-electronic-prescribing-of-controlled-substances-request-for-information-rfi.
- Electronic Prescribing of Controlled Substances (EPCS) among Office-Based Physicians, 2017. HealthIT.gov. Updated September 2019. Accessed September 16, 2021, https://www.healthit.gov/data/data-briefs/electronic-prescribing-controlled-substances-epcs-among-office-based-physicians.
- The SUPPORT for Patients and Communities Act (P.L.115-271): Medicare Provisions. In: Service CR, editor. R45449. January 2, 2019 ed2019.
- CMS Delays Federal EPCS Compliance Enforcement To 2022. RXNT. Updated February 17, 2021. Accessed August 9, 2021, https://www.rxnt.com/cms-delays-epcs-compliance-enforcement-to-2022/.
- CMS formally delays EPCS enforcement. National Community Pharmacists Association. Updated December 4, 2020. Accessed August 22, 2021, https://ncpa.org/newsroom/qam/2020/12/04/qam-ad-cms-formally-delays-epcs-enforcement.
- CMS delays enforcement of e-prescribing requirement for controlled substances. California Medical Association. Updated December 15, 2020. Accessed September 16, 2021, https://www.cmadocs.org/newsroom/news/view/ArticleId/49150/CMS-delays-enforcement-of-e-prescribing-requirement-for-controlled-substances.
- State PDMP Profiles and Contacts. Prescription Drug Monitoring Program Training and Technical Assistance Center. Updated July 7, 2021. Accessed August 19, 2021, https://www.pdmpassist.org/State.
- SB19-079 Electronic Prescribing Controlled Substances. Colorado General Assembly. Updated April 8, 2019. Accessed September 17, 2021, https://leg.colorado.gov/bills/sb19-079.
- Illinois HB3596 Controlled Substances–Opioids. Trackbill. Updated August 20, 2021. Accessed September 17, 2021.
- What is a PDMP (Prescription Drug Monitoring Program)? Practice Fusion. Updated September 1, 2021. Accessed September 16, 2021, https://www.practicefusion.com/blog/what-is-a-pdmp/.
*Electronic Prescriptions for Controlled Substances (EPCS) is only available in the 50 US states and the District of Columbia
